VIVAN Life Sciences Offers wide range of Impurities / Pharmaceutical Drug Impurities. Impurities are unwanted chemicals that remain with the active pharmaceutical ingredients (APIs) or develop during formulation or upon aging of both API and formulation. The presence of these unwanted chemicals even in trace amount may influence the efficacy and safety of pharmaceutical product. The control of impurities is an important task pharmaceutical impurities as per the regulatory norms. High Pure and Well characterized impurity Standards are used for Related Substances, Organic impurities and Validation of Analytical Methods.
All the Impurity standards are provided with CoA, MASS, NMR & HPLC reports, conforms to Global quality standards.

VLIM-00077
174227-14-6
C₂₅H₂₅N₅O₅S
507.565

VLIM-00078
6292-59-7
C₁₀H₁₅NO₂S
213.3

VLIM-00079
86404-63-9
C₁₆H₁₄F₃N₅O
349.31

VLIM-00080
182369-73-9
C₁₆H₁₅F₂N₅O
331.32
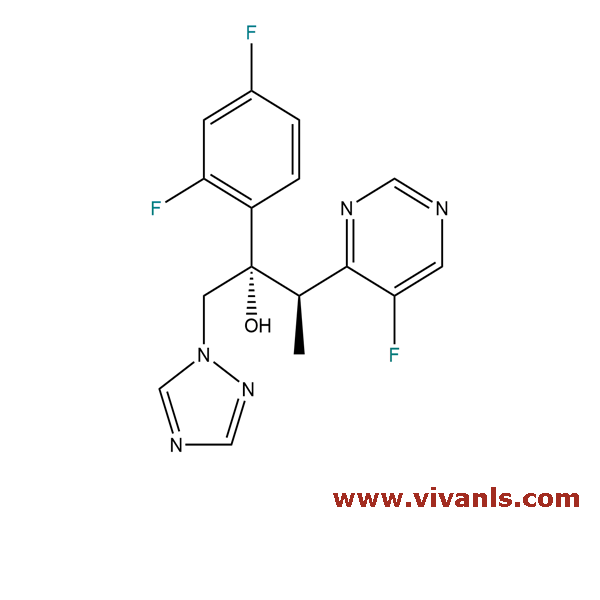
VLIM-00081
137234-63-0
C₁₆H₁₅F₂N₅O
331.32

VLIM-00082
5233-42-1
C₇H₇Cl₂N₃O₄S₂
332.18

VLIM-00083
144750-52-7
C₁₆H₁₆ClNO₂S.H₂SO₄
419.9

VLIM-00084
120202-71-3
C₁₆H₁₆ClNO₂S.H₂SO₄
419.9

VLIM-00085
5796-17-8
C₉H₁₁NO₄
197.19
-1664176505.png)
VLIM-00086
33968-67-1
C₉H₂₃BrN₂
239.2
-1664176564.png)
VLIM-00087
2247938-20-9
C₁₉H₄₃BrN₂
379.46
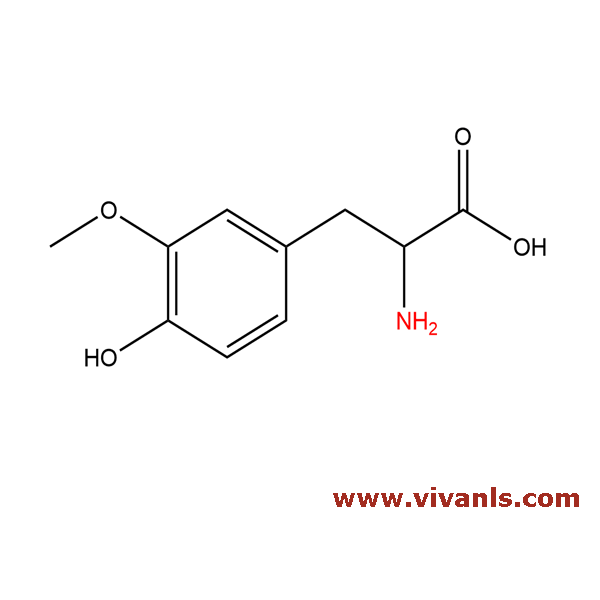
VLIM-00088
7636-26-2
C₁₀H₁₃NO₄
211.21